Support IICT Call
Support for submissions to the SNSF Investigator-Initiated Clinical Trials (IICT) program
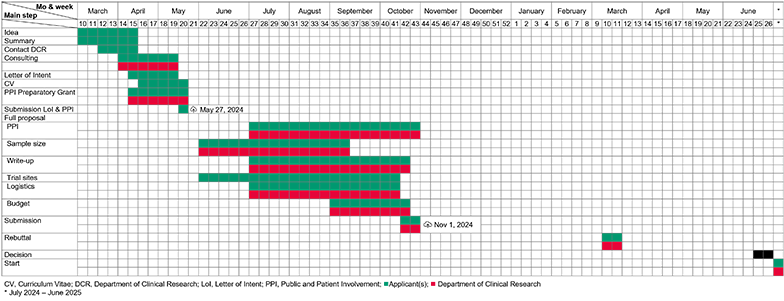
The Investigator-Initiated Clinical Trials program of the Swiss National Science Foundation is a funding scheme targeting clinical researchers who wish to conduct a multicenter randomized-controlled trial which is beyond the normal Swiss National Science Foundation project funding scheme in terms of its research question, comprehensive nature, duration, complexity, and costs. More information about the program can be found on the program website. The whole process follows a five-step approach (for details see below):
- Call for proposals
- Submission of a letter of intent
- Submission of full proposal
- Rebuttal to written peer reviews
- Decision by an independent panel
Preparation of the grant proposal is also different to the preparation of a proposal for normal project funding i.e., involves more people including the public or patients, requires more time, and additional preparatory work. The Department of Clinical Research (DCR, including CTU and CIU) encourages clinical researchers to consider a submission and supports submissions for this funding scheme.
Due to the number of submissions each year and the amount of work and time required to prepare a high-quality proposal; we ask applicants to follow the below described process. Otherwise, we cannot guarantee appropriate support and even might not be able to support an application at all. Please also take note, that you must contact us until May 1st if you plan to submit a proposal. Otherwise, we cannot guarantee appropriate support.
Idea
Develop the project idea and discuss with colleagues. The program is dedicated to large, multicenter randomized-controlled trials. The majority of participants should be enrolled within Switzerland (with some exceptions) at more than two sites but non-Swiss sites are allowed. The intervention to be tested can be treatment(s), preventive intervention(s), screening procedure(s), or diagnostic(s). Previous budgets ranged from a few hundred thousand CHF to 3.4 Mio. Treatment costs are eligible e.g., for the production of the Investigational Medicinal Product.
Non-randomized, pilot, or proof-of-concept trials are not eligible as well as observational or preclinical studies.
Summary
Once the project idea has become clear, we suggest to draft:
- A summary based on the PICO scheme (Population, Intervention, Control, Outcome(s))
- A first version of the trial flow chart
- A rough study schedule table
This serves for further discussions and to clarify the main objectives.
Contact DCR
Once you have the summary ready it is time to contact the Department of Clinical Research for a first discussion (consulting). To do so, please send an e-mail with all what you have so far (especially summary, draft flow chart and study schedule)
until May 1st, 2024 latest
to info.dcr@unibe.ch
(cc any person from DCR/CTU/CIU that you were already in contact with)
Otherwise, we cannot guarantee appropriate support and even might not be able to support an application at all.
Consulting
During the first consulting session, we will discuss the study question, trial design aspects, and feasibility as well as whether the project fits to the call.
Based on the number of project ideas, DCR support for proposal development can vary.
Letter of Intent
A letter of intent has to be submitted to the Swiss National Science Foundation until May 27th. The letter needs to use the dedicated template. The letter itself is not peer reviewed. Its primary purpose is to facilitate selection of peer reviewers by the Swiss National Science Foundation and to allow a formal eligibility check of the applicants. Therefore, the general study population and the experimental interventions to be evaluated are fixed, once the letter is submitted. Other aspects such as the sites, the sample size, or the budget are only indicative and can be adapted in the full proposal.
If a clinical trials unit is involved and planned to act as partner, a letter of support must also be submitted.
CV
A curriculum vitae must be prepared using the dedicated template of the Swiss National Science Foundation. More information can be found on the CV website. If you have not used the format so far, plan for enough time to enter all the information on the website (no Word template or similar). We suggest to do it at least 10 days before submission deadline.
PPI Preparatory Grant
Active involvement of patients, members of their family, carers, the public, or the relevant patient organizations (Patient and Public Involvement) is mandatory across the entire lifecycle of the project (development, set-up, conduct, and completion). To allow for appropriate planning of these activities, Swiss National Science Foundation provides a preparatory grant of up to CHF 5,000. The grant covers Patient and Public Involvement activities for developing the full proposal including the plan for activities over the course of the project.
The preparatory grant proposal is primarily a budget justified by a patient engagement plan. The budget can cover compensation of PPI representatives for the time spent on providing input, as well as reimbursement of travel costs and expenses for accommodation and meals. In addition, the costs associated with the organization of meetings for PPI activities can be charged to this grant.
The proposal is not sent out for peer reviewer but is evaluated by the Swiss National Science Foundation internally to ensure that formal requirements are met.
Submission LoI & PPI
The Letter of Intent and the PPI Preparatory Grant proposal have to be submitted to the Swiss National Science Foundation until May 27th, 2024 17:00 via mySNF (not the SNSF Portal).
Full Proposal
Developing the full proposal is labor intensive. It not only requires dozens of working hours but also time for repeated discussions and networking activities. The Department of Clinical Research can support you in various activities.
Writing of the full proposal
Writing the full proposal usually requires more work than the standard project grant proposal. Therefore, plan enough time and start early! Department of Clinical Research provides support for writing grant proposals. We also have templates for specific parts of the proposal. For those, you do not need to write anything, this will be done by us. Responsibilities will be defined once the letter of intent is submitted.
Please do not forget that it is important to document how the Patient and Public Involvement activities in the development of the full proposal influenced the proposal.
Patient and Public Involvement activities
In the research plan, applicants must document their efforts and plans to actively involve patients, members of their family, carers, the public, or the relevant patient organizations across the entire lifecycle of the project (from the design of the study to its management and conduct, data analysis, dissemination of results and final evaluation). During the development of the full proposal, activities focus on two main areas:
- Getting input to the clinical trial itself
- Developing a Patient and Public Involvement activities plan for the project
The Department of Clinical Research will support you in these activities.
Sample size calculation and statistical study design
Sample size is often critical for the feasibility and success of a clinical trial. The Department of Clinical Research can provide you with the appropriate sample size calculation. Note that this is usually not a one-off calculation but rather an iterative process where we provide several scenarios and where changes to the design (can) happen. The amount of work varies a lot, from a few hours too dozens of hours. Please take this into account.
Contacting potential trial sites and collection of Letters of Commitment
Start very early to contact potential trial sites. They will need to provide a letter of commitment. The letter must contain evidence for the site-specific enrolment potential. This evidence can come from pilot studies, previous trials (of similar population and interventions) or extracts from the data warehouse of the hospital/institution (preferably multiple sources). Simple statements about enrolment potential are not sufficient!
Logistics and collecting offers (especially in case of trials with medicinal products)
Especially for trials with medicinal products (medical devices), explore different options for the supply. The grant can cover these costs. Placebo production can be expensive, so different suppliers should be approached. You will need formal offers for the submission. We can help identifying suppliers and support you in the discussions with the suppliers.
Budget
The budget for the IICT Call is different to the normal project funding budget. It has much more details. Plan enough time for entering in mySNF. Applicants can ask for money to employ personnel. We suggest that this is restricted to personnel for management and coordination of the project. Costs related to participants care should be budgeted as “patient fee”, usually also for the site of the applicants. Patient fees can also be paid to non-Swiss sites. The budget must be split in yearly tranches. These are primarily of relevance for the administration/accounting of the Swiss National Science Foundation. If in doubt, budget activities rather too early than too late. The budget for CTU activities requires a special format. We provide this to you. Department of Clinical Research will support you in the development of the budget including the justification for the patient fee (which is required).
Submission
Submission of the full proposal is via mySNF. Submission of an IICT grant proposal is more extensive than for normal project funding. We strongly suggest to start the submission process at least two weeks before the submission deadline (Nov 1st, 2024 17:00) i.e., start mid October.
Once your proposal is submitted, we will fix two dates for meetings to discuss the rebuttal (see below).
Rebuttal
All proposals that meet formal requirements (checked by Swiss National Science Foundation internally) are sent out for international peer review. From experience, you will receive at least four written peer reviews one of which is from a biostatistician.
The Swiss National Science Foundation will forward you the reviews beginning of March 2025 and asks you to provide a rebuttal letter within two weeks. This rebuttal should be a point-by-point discussion of the points raised by the peer reviewers. Note that no changes to the proposal are possible at this stage. The rebuttal can only clarify misunderstandings or provide further explanations and justifications for certain design choices.
We suggest to have at least two meetings for the rebuttal: one at the beginning of the two weeks to go through the comments, discuss possible responses, and distribute responsibilities for drafting the response to the different points, and one at the end of the two weeks to finalize the letter.
The rebuttal letter must be submitted via mySNF (not the SNSF Portal).
Decision
The full proposals with the accompanying peer reviews and the rebuttal are discussed by a dedicated, international panel of clinical research experts (clinical researchers, biostatisticians, (clinical) epidemiologists, …). The panel provides recommendations to the Research Council which makes the funding decisions.
The decision will be communicated in writing during the second half of June 2025.
Start
If your project is funded, the official start of the funding period must be between July 2025 and June 2026. Activities (e.g., drafting the trial protocol or other documents) can be started even before the official start of the funding period given that you have enough resources.