The SWISS-APERO trial
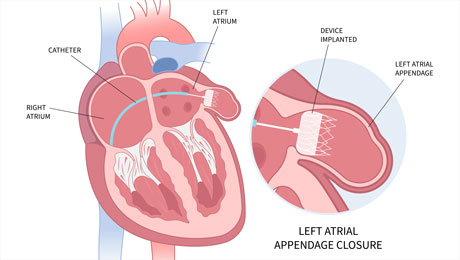
02.11.2023 – Results from the one-year follow-up of the SWISS-APERO trial, a randomized trial of patients with non-valvular atrial fibrillation who required percutaneous left atrial appendage closure, have been released.
This trial is the first of its kind to compare the Watchman FLX device (manufactured by Boston Scientific, USA) and the Amplatzer Amulet device (produced by Abbott, USA). These are the two primary devices used globally for left atrial appendage closure.
Out of the 221 patients in the study, 111 (50.2%) were assigned to Amulet, while 110 (49.8%) to Watchman. For those patients where the left atrial appendage anatomy was suitable for either the Amulet or Watchman devices, there was no significant difference in rates of Patent Appendage and Device Related Thrombus at the 13-month Cardiac Computed Tomography Angiography. Likewise, the clinical outcomes were comparable between the two groups.
CTU Bern was heavily involved from planning to publication of the trial; CTU Bern was responsible for the full statistical analysis process (Statistics and Methodology).
The relevant publication can be also found here: https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.123.067599?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed

