News

DCR Medical Grant Writing Program for Clinicians
18.04.2024 – The Department of Clinical Research is offering a Medical Grant Writing Program for Clinicians who would like to enhance their skills regarding applications for grant funding for clinical research projects.
Registration is open until May 1st.
For more information please see attached flyer and go to: https://www.dcr.unibe.ch/resources__training/dcr_medical_grant_writing_program_for_clinicians/index_eng.html
Flyer: DCR Medical Grant Writing Program for Clinicians
Open Science – Courses offered by the University Library of Bern
14.03.2024 – The Data Stewards at the Open Science Research Data Management Team offer individual support and training sessions as well as workshops based on your needs for you and your team on request.
Read more here.

SCTO Symposium 2024
11 June 2024, 10.00–16.15
AGORA | Paternot auditorium | Lausanne
Working towards efficient clinical data-driven research in Switzerland
This year’s SCTO Symposium is dedicated to the topic «Working towards efficient clinical data-driven research in Switzerland» and will take place in Lausanne on Tuesday, 11 June.
For more information and to register click here.

SNSF IICT Call 2024 open – new process at DCR
04.03.2024 – The IICT program is targeted at researchers who wish to conduct an investigator initiated clinical trial. The Department of Clinical Research offers extensive support for developing proposals for this funding scheme.
Please contact us until April 14th for a consulting so we can support you in developing your study idea: Info.dcr@unibe.ch
More information on the call and the whole process can be found here.
New seminar series: Facts and pitfalls of observational studies – How to plan and conduct HRO projects
27.02.2024 – Almost 80% of projects submitted to the ethics committees are not clinical trials, but human research projects falling under the HRO (Human Research Ordinance). These are observational studies and further use projects, and as the range of possible projects is large, it is often challenging to keep track of qualitative, regulatory and legal requirements. A new training format offers help to navigate the project-jungle: The HRO lunch sessions provide practical and concise information about main topics related to HRO projects and offers plenty of room for researcher’s questions. More information and the links for registration can be found here.

DCR-CTU Lecture Dezember 2023: Tackling Lasagna’s law: perspectives on clinical trial enrolment from three recent projects
Mittwoch, 13. Dezember 2023
12.45-13.30 Uhr (via Zoom-Meeting)
«Tackling Lasagna’s law: perspectives on clinical trial enrolment from three recent projects»
Referierende:
- PD Dr. med. Manuel Blum, Universitätsklinik für Allgemeine Innere Medizin, Inselspital
- Prof. Dr. med. Daniel Fuster, Universitätsklinik für Nephrologie und Hypertonie, Inselspital
- Dr. phil. Anna Schöni, Berner Institut für Hausarztmedizin (BIHAM), Universität Bern
Weitere Informationen zu den Lectures und der Registrierung finden Sie hier.

Der Cannabispilotversuch SCRIPT eröffnet Anmeldung zur Studienteilnahme
04.12.2023 – Nachdem das Bundesamt für Gesundheit (BAG) und die zuständigen Ethikkommissionen der SCRIPT-Studie im Frühling 2023 grünes Licht erteilten,

Demonstration: reaction-Studie, sitem-insel «Tag der offenen Tür» 2.12.2023
Am «Tag der Offenen Tür» führt das DCR mit seinen Units CTU und CIU die reation-Studie durch. Sie dient als Demonstration zur Veranschaulichung einer randomi-
sierten kontrollierten Studie.
Melde dich zur Studienteilnahme und finde heraus, ob der reaction-Drink dir Power verleiht – frei nach dem Motto «nicht besser, aber schneller»!
Samstag, 2. Dezember 2023, «Tag der offenen Tür» sitem-insel
Department of Clinical Research, 1. OG Ost
Studienteilnahme: 10.00 − 14.00 Uhr, Auswertung laufend bis 16.00 Uhr
Für weitere Infos bitte hier klicken.

New members of staff: Samara and Johanna
19.11.2023 – We are pleased to welcome Samara Naim as a new staff member at CTU Bern.
Samara completed her PhD in Biomedical Sciences at the Institute of Pharmacology at the University of Bern and thereafter worked in the quality department at CSL Behring in Bern, where she was mainly involved in the deviation management of final pharmaceutical products. After conducting basic research and quality evaluations of final products, she now wishes to learn as much as possible about one of the intermediate steps during the development of new medicinal products – clinical trials. Therefore, she is very excited to start her new role as Clinical Trial Monitor.

We are also pleased to welcome Johanna Lippuner as a new staff member at CTU Bern. Johanna is currently pursuing her bachelor’s degree in psychology at the University of Bern. Previously she worked for a management consultancy firm in Zürich.
Now she is looking forward to learning more about clinical research and expanding her skills in her position as a Junior Research Assistant in the Clinical Data Monitoring team.
Welcome Johanna!
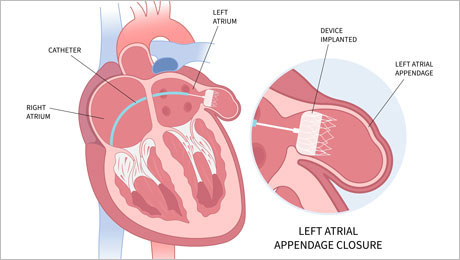
The SWISS-APERO trial
02.11.2023 – Results from the one-year follow-up of the SWISS-APERO trial, a randomized trial of patients with non-valvular atrial fibrillation who required percutaneous left atrial appendage closure, have been released. Click here for more information.

The BIOSTEMI ES study
02.11.2023 – Final five-year follow-up results of a randomized trial in patients with ST elevation Myocardial infarction (STEMI) in need of catheter-directed revascularization have now been published.
Find out more here.
Study Talk @ Inselspital
Der nächste Znüni Talk steht bevor.
Zielpublikum: Study-Nurses, Studienkoordinator:innen, Wissenschaftliche Mitarbeitende und Clinical Research Coordinators aus dem Inselspital
Dienstag, 14. November 2023
9.00 bis 10.00 Uhr, Auditorium Maurice E. Müller, Operationstrakt Ost (OpO)!
Titel: «ORCA – QM-Applikation als Support für die Qualitätssicherung in der Forschung»
Referent: Michael Lensch, Fachexperte Qualitätsmanagement, Inselspital, Universitätsspital Bern, Direktion Lehre und Forschung
Anmeldung: bis 9. November 2023 unter: studytalk@insel.ch

DCR Newsletter Oktober 2023
30.10.2023
- Neues DCR-Logo
- DCR-Grant Writing Workshop for Clinicians
- Schreiben des Studienprotokolls – eine kleine Auswahl an Dos und Don’ts (Teil 1)
- CTU AGBs Reloaded: Neue Terms and Conditions dank optimierten internen Prozessen
- CIU – Studie menuCH-Kids
- CTU Kurstermine

6th SPCRC Congress
10.10.2023 – The Swiss Professionals of Clinical Research Coordination (SPCRC) invites you to the 6th congress:
Friday, 3 November 2023, 9.00 – 17.00
Hotel Mövenpick, Basel, Aeschengraben 25, 4051 Basel
To access the invitation and the detailed program, please click here.

DCR-CTU Lecture: Genetics for Precision Drug Development
Mittwoch, 25. Oktober 2023
12.45-13.30 Uhr (via Zoom-Meeting)
Titel: Genetics for Precision Drug Development
Referent: Vincent Mooser, MD, Canada Excellence Research Chair in Genomic Medicine, Department of Human Genetics, McGill University, Montreal QC
Please find more information about the lectures here.

Update to T&C: New Terms and Conditions due to optimized internal processes
10.10.2023 – We invite you to read about our updated terms and conditions for CTU Clinical Research Agreements regarding new information on data management, respectively database responsibilities and fees. To access the details, please click here.

International research initiatives led by University of Bern receive funding
03.10.2023 – The Swiss National Science Foundation (SNSF) has awarded funding to three projects led by the University of Bern as part of the SPIRIT program, promoting cross-border and collaborative research endeavors. Please click here to read more.

DCR-CTU Lecture: Prognostische Modelle in der klinischen Forschung – Ein Blick in die Glaskugel
Mittwoch, 27. September 2023
12.45-13.30 (Zoom Meeting)
Referent: André Moser, Senior Statistician, CTU Bern
Die Vortragssprache ist Deutsch.
Study Talk @ Inselspital
Der nächste Znüni Talk steht bevor.
Zielpublikum: Study-Nurses, Studienkoordinator:innen, Wissenschaftliche Mitarbeitende und Clinical Research Coordinators aus dem Inselspital
Dienstag, 5. September 2023
9.00 bis 10.00 Uhr, Kinderklinik A, Kursraum 1, Inselspital
Titel: «Umgang mit vulnerablen Personen in der klinischen Forschung»
Referent: Prof. em. Dr. med. Christian Seiler, Präsident der Kantonalen
Ethikkommission Bern (KEK)
Anmeldung: bis 1. September 2023 unter: studytalk@insel.ch
Courses offered by the University Library of Bern, Open Science
21.07.2023 – The Open Science Research Data Management Team from September 2023 offers new “How to” training series in research data management.
They offer individual support and training sessions as well as workshops based on your needs for you and your team on request.
Please click here for more information, the registration links, and the flyer.
Apéro for Females in Clinical Research
21.07.2023 – The Department of Clinical Research invites you to a special Apero event dedicated to women undertaking translational and clinical research. This informal gathering aims to provide an opportunity to connect, share experiences, and build valuable networks with colleagues at the University of Bern and University Hospitals.

DCR Newsletter Juni 2023 (früher CTU Newsletter)
27.06.2023
- Die DCR-Mitarbeitenden kurz vorgestellt...
- Patient and Public Involvement (PPI)
- Easy Guide to Clinical Studies – ein neues Online Tool der SCTO für klinisch Forschende
- CIU – BEready Studie
- CTU-Kurstermine

SERVE trial finds no effect of tadalafil in patients with systemic right ventricles
15.06.2023 – Final results of a double-blind, placebo-controlled, randomized trial in patients with a rare, congenital heart disease have now been published.
Find out more here.

DCR-CTU Lecture: The Power of Patient Engagement: Patient and Public Involvement (PPI) in Clinical Research
Mittwoch, 28. Juni 2023
12.45-13.30 (Zoom Meeting)
Referierende:
- Prof. Eva Segelov, Director of the Department of Clinical Research; - Sarah Berner, Project PPI, Department of Clinical Research; - Ein Forscher und ein Mitglied des DCR Patient Panel
Optimising prescribing in older adults with multimorbidity and polypharmacy in primary care
06.06.2023 – The Optimising PharmacoTherapy In the multimorbid elderly in primary CAre (OPTICA) trial studied the effects of a primary care medication review intervention in older adults with multimorbidity and polypharmacy. Final results are now presented in the BMJ. Click here to read more.
TARGET-READ: results published
06.06.2023 – Results of a randomized clinical trial on the effects of a multimodal transitional care intervention in patients at high risk of readmission were published in JAMA Internal Medicine. Click here to read more.
Znüni Talk @ Inselspital
Der nächste Znüni Talk steht bevor.
Zielpublikum: Study-Nurses, Studienkoordinator:innen, Wissenschaftliche Mitarbeitende und Clinical Research Coordinators aus dem Inselspital
13.06.2023 «Patient and Public Involvement in der klinischen Forschung»
Referentinnen: Tamara Kohler, PPI Project Manager, Swiss Clinical Trial Organisation (SCTO) / Sarah Berner, PPI Project Officer, Department of Clinical Research (DCR)
09:00 Uhr bis 10:00 Uhr, Kinderklinik A, Kursraum 1, Inselspital
Anmeldung: bis 9. Juni 2023 unter: studytalk@insel.ch
ELAN trial shows: early initiation of treatment with blood thinners after stroke is safe and possibly more effective
26.05.2023 – The international randomized ELAN trial aimed at estimating the effect of early initiation
as compared with late initiation of anticoagulation in patients suffering from ischemic stroke with atrial fibrillation. Results have now been published in the New England Journal of Medicine and question recommendations in current guidelines. Click here to read more.

DCR Medical Grant Writing Program for Clinicians
03.05.2023 – The Department of Clinical Research is offering a Medical Grant Writing Program for Clinicians who would like to enhance their skills regarding applications for grant funding for clinical research projects.
Registration is open until May 19th. Application decisions can be expected by May 23rd.
For more information please see attached flyer and go to: https://www.dcr.unibe.ch/events/dcr_medical_grant_writing_program_for_clinicians/index_eng.html.
CAS Clinical Reserach Coordinator
03.05.2023 – Register now for the CAS Clinical Research Coordinator which will start in August 2023. Click here to read more.

Day of Clinical Research – Save the Date!
17.03.2023 – The Day of Clinical Research will take place in the week of Clincial Research and is organized by the Department of Clinical Research (DCR).
Thursday, 7 December 2023, 12.00-18.00
Langhans Auditorium, Inselspital, Bern
Please find more information about the workshops and keynote speakers in the flyer below.
New study questions standard therapy for preventing kidney stones
06.03.2023 – Kidney stones are a common condition, about 10% of the population is affected. Diuretic drugs from the class of thiazide diuretics have been considered as the treatment of choice for the prevention of kidney stone recurrence for decades.
A new study now published in the journal The New England Journal of Medicine calls this therapy into question. CTU Bern was involved in the planning, data management, monitoring, project management and statistical analyses of the trial. For more information click here.

Weiterbildungskurs klinische Studien: Grundlagen Qualitätsmanagement
02.03.2023 – Register now for this training course, which will be held in German on 12 September 2023. Click here to read more.

CTU Newsletter Februar 2023
28.02.2023
- Spnosor vs. Sponsor-Investigator vs. Investigator
- Datensicherung
- Nächste Kurstermine

Work Shadowing at DCR - fully booked
09.02.2023 – Twice a year, in spring and autumn, the Department of Clinical Research (DCR) offers a five-day work shadowing.
During these five days, participants gain an insight into Data Management, Monitoring, Statistics, Clinical Study Management, Quality Management and the work of the Clinical Investigation Unit. The offer is primarily aimed at people who have to complete an internship as part of their CAS course (e.g. CAS Clinical Research or CAS in Clinical Trial Management). The next work shadowing will take place from 27 to 31 March 2023, where two places are still available. If you are interested, please contact info@ctu.unibe.ch
Study published on the effect of antimicrobial treatment duration
01.02.2023 – A new study from Switzerland investigated the effect of antimicrobial treatment duration on in-hospital mortality in critically ill patients.
There was no evidence for a survival difference between patients who received a shorter treatment (<10 days) vs a longer treatment (more than 10 days). The study used retrospective data from two tertiary care intensive care units in Switzerland (Inselspital Bern and CHUV Lausanne). CTU Bern was involved in the statistical analysis of the study. More information can be found here.