News 2018

CTU Newsletter Dezember 2018
• Data Sharing in der Medizin
• News von den SCTO-Plattformen
• Die CTU stellt sich vor: Data Management
• Nächste Kurstermine

MASTER DAPT trial: 1850 patients enrolled
Up to now, 1850 high bleeding risk (HBR) patients with coronary artery disease (CAD) have been successfully enrolled in the MASTER DAPT trial in 96 sites around the world. Click to read more.

Transparenz
Eine vor kurzem im BMJ publizierte Studie hat aufgezeigt, dass weniger als 50% der klinischen Versuche, welche im EU Clinical Trials Register (EUCTR) registriert sind, ihre Ergebnisse öffentlich zugänglich machen.
Diesem Thema nimmt sich nun ein Feature im Wissenschaftsmagazin des SRF2 an. Mehr lesen.

Fundamental Concepts in Epidemiology
This course will provide an orientation to health and medical research from a quantitative and epidemiological viewpoint. It will give an introduction to the design of public health and clinical research, and it will discuss measures of disease frequency and association, and the validity of research in medicine. The course will discuss recent developments in epidemiologic methods for public health and clinical research. It will review the various study designs and major issues in the validity of epidemiologic studies. It will give an overview of elements of epidemiologic data-analysis.
Please find here the link for the course registration.
The program of the course can be found here:

CTU Newsletter September 2018
- Datenschutz
- Umgang mit Daten
- Rechte der Studienteilnehmenden
- Widerruf der Einwilligung
- Anonymisieren der Daten
- Löschen der Daten
- General Data Protection Regulation
- News von den SCTO-Plattformen
- Nächste Kurstermine
Three cardiology trials came to a conclusion
Three large randomized cardiology trials came to a conclusion recently: the GLOBAL LEADERS, MATRIX and Bioscience Orsiro trials. CTU Bern was involved in all three trials. Click here to read more.

The BISS trial: Recruitment now open!
Botox Instead of Strabismus Surgery

The second national Congress for Study Nurses and Research Coordinators will take place at the NH Hotel in Fribourg on Friday, October 26th, 2018.
To view the program and to find out how to register,
click here.
The Federal Council submitted a draft legislation article for cannabis pilot trials for consultation
16.07.2018 – The Federal Council submitted a draft legislation article for cannabis pilot trials for consultation. This article and a corresponding act regulates the conduct of scientific studies in the field of recreational cannabis use. The article does not affect regulations of cannabis use outside of such scientific studies. Read more

CTU Newsletter Juni 2018
- News von den SCTO-Plattformen
- Das Quality Management stellt sich vor
- Nächste Kurstermine

Data Management Plan: Writing Labs & Individualized Consulting
Since October 2017, researchers have to submit a data management plan (DMP) to the SNSF in the course of a grant application. The University of Bern offers a set of services for everyone who needs to write an SNSF Data Management Plan for the October deadline. Click here to find out more about the support services.

New project website released
Do you know hereditary thrombotic thrombocytopenic purpura (hTTP)?
Likely not, because hTTP is an ultra-rare disease, affecting only around one person out of a million. In Bern at the Inselspital, one of the largest patients cohorts of this disease, the Hereditary TTP Registry, is maintained. To reach out to more patients and their clinicians to join the Hereditary TTP Registry, a new public website has been released recently.
Click to read more.
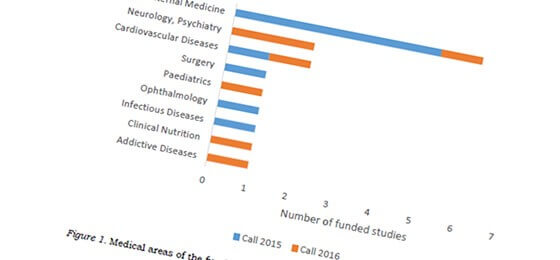
Evaluation of second IICT call: intensive preparations required
In 2015, the SNSF has started a special program to promote Investigator Initiated Clinical Trials (IICT). The funding for IICTs continues to be in high demand. In the recently published evaluation of the 2016 call, the SNSF addresses main reasons for the rejection of applications. Click to read more.
PragMagic Online Tool
The PragMagic Tool aims to support trial design teams to maximize generalizability of trial findings to the routine care setting of interest while ensuring validity and operational feasibility of the trial. It has been developed with the support of CTU Bern and the Insititute for Social and Preventive Medicine of the University of Bern.
Read more.
New study published
A prospective multicenter cohort study, supported by CTU Bern, has shown that elderly patients with acute venous thromboembolism have a substantial long-term risk of recurrent venous thromboembolism and that recurrence carries a high case-fatality rate. Only two factors were independently associated with recurrent venous thromboembolism. Read more.

BERN TMVI
Mitral regurgitation is a frequent condition leading to relevant mortality and morbidity in elderly patients. More information can be found here.
Cardiology Trial: Recruitment open!
EVOPACS
Reduction of low-density lipoprotein cholesterol (LDL-C) levels effectively reduces the risk of adverse events in patients with established atherosclerotic cardiovascular disease. Click here to read more.

2. D|A|CH Symposium in Zürich
Der 2. Dreiländerkongress für Klinische Prüfungen findet am 11. & 12. Juni 2018 in Zürich statt. Der von den Koordinierungszentren für Klinische Studien in Deutschland, Österreich und der Schweiz (D-A-CH) organisierte Kongress, richtet sich an alle, die im klinischen Bereich tätig sind.
Klicken Sie hier für weitere Informationen.

New version of CDISC Glossary released
The CDISC glossary is a comprehensive resource for definitions related to clinical research. It is based on authoritative sources and contains definitions of over 650 terms. The newest version of the CDISC Glossary is linked to the National Cancer Institute NCI Thesaurus. Click to read more.

Start of second cohort in April 2018
The sitem-insel School aims to promote researchers and train executives in the fields of translational medicine and biomedical entrepreneurship.
The second cohort of students will start on 19 April 2018. Interested candidates have the chance to submit their application until the end of March.

